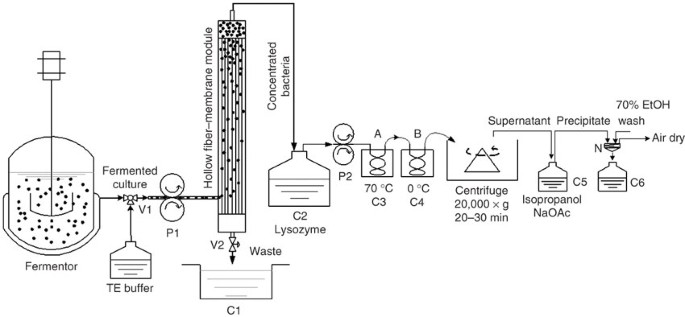
This protocol describes a streamlined method of plasmid DNA extraction by continual thermal lysis, a modification of the basic boiling lysis technique, to simplify the processing of large volumes of Escherichia coli cultures. Fermented bacteria are harvested using a hollow fiber-membrane module and pre-treated with lysozyme prior to passing through a thermal exchange coil set at 70 °C to lyse the cells, and into a juxtaposed cooling coil on ice. The lysed and cooled bacteria are subsequently separated from the lysate by centrifugation and plasmid DNA is precipitated from the supernatant for further purification. The use of peristaltic pumps and two heating coils at constant temperature without the use of centrifugation enable the lysis process to become constant and controllable, providing a flow-through protocol for cell lysis and plasmid DNA extraction. Large volumes of bacterial cultures (20 l) can be processed in 2 h, yielding approximately 100 mg plasmid DNA l −1 culture, making this an attractive protocol for consistent and large-scale preparation of plasmid DNA.


Recent progresses in the development of DNA vaccination and gene therapy show great potential for the treatment of disease. As clinical application of these technologies will require large quantities of plasmid DNA at the milligram level, there is an increased demand for large quantities of recombinant plasmid DNA 1,2 . Thus, developing a large-scale, reproducible and cost-effective protocol for plasmid DNA preparation is now one of the most challenging tasks in DNA vaccine development 3,4,5 .
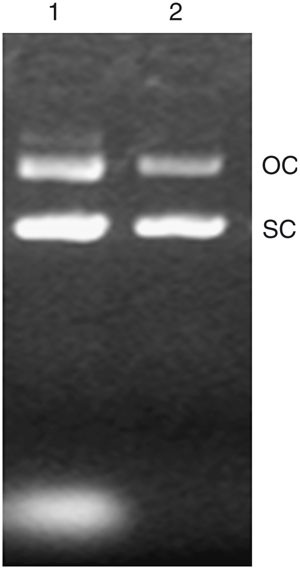
Target DNA sequences can be amplified to produce large quantities of purified DNA for use in such applications as gene therapy by ligation into a plasmid vector, which can then be transformed into suitable bacterial cells. These recombinant bacterial strains will replicate the plasmid, producing large quantities of the target DNA 6 . A typical process of plasmid DNA generation includes the construction and selection of appropriate expression vectors, production of recombinant microorganisms and cell growth, followed by lysis of the cells, and the extraction and purification of plasmid DNA 7 . When the plasmids are extracted and purified, they usually result in one of two forms: either open-circled or supercoiled. However, when they are used in gene therapy or vaccine immunization, the plasmid with the supercoiled form is preferred. During plasmid DNA extraction, the step of lysing bacterial cells without losing plasmids is probably the most critical for downstream processing 8 , as the quantity of plasmid DNA recovered is mostly determined by this step after fermentation 9,10 .
Plasmid DNA extraction from bacterial cells usually begins with a chemical lysis step 6 , such as alkaline lysis 11 , which is a widely adopted method in research laboratories. However, during alkaline lysis, precipitates form that contain cell debris, denatured proteins and nucleic acids, which must be removed. Centrifugation on a fixed-angle rotor is the most common operation during this process 12,13 and is a rate-limiting step for a large-scale preparation 14 . An alternative method is boiling lysis, in which the 100 °C thermal treatment disrupts and makes cells release the plasmid DNA 15 . Although boiling lysis can isolate more plasmids than alkaline lysis, the inconsistent plasmid yield and purity, and the difficulty in operation, make this method undesirable even for laboratory research-scale preparations 16 . For large-scale production, a high-pressure homogenizer is used to continuously disrupt cells 17 . However, this often causes problems in collecting fragmented DNA plasmids and more contaminated genomic DNA due to its severe fluid dynamic forces for destruction 17 . Therefore, the development of a highly efficient continuous lysis and scalable protocol becomes a challenging but important issue.
Based on the conventional boiling lysis protocol 18 , Lee and Lander have made several modifications to improve the method 19,20 . Although these modifications significantly improve the efficiency of boiling lysis and the yield of plasmid DNA, they require centrifugation steps at the bacterial-collecting and plasmid-extracting stage, special equipment and reagents. In a recent report 21 , we described a design for a flow-through protocol from cultured bacteria for the extraction of plasmid DNA. The schematic design of the device and set-up is shown in Figure 1. The use of a 0.2 μm pore size hollow fiber-membrane module to harvest the fermented bacteria will minimize the centrifugation step, which is one of the limiting steps in large-scale preparation. Pumping through the heat-exchanging coils, cells receive uniform thermal treatment regardless of the volume of the sample, which ensures continuity and homogeneity of cell lysis. The diameter of copper coils can vary, depending on the volume of fermentation. This provides versatility for any given volume of samples. This protocol does not aim to increase the yield of plasmid, but to provide consistency between batches, which can otherwise be lacking in boiling lysis methods. A centrifugation step is, however, still required to separate the plasmid-containing supernatant from the lysed bacteria, which will require further optimization. Overall, this protocol provides a scalable, efficient and cost-effective preparation system to meet the needs of large-scale plasmid production for both laboratories and industry. The protocol mainly details the lysis of cells and the extraction of plasmid DNA, and although further purification procedures may be required, they are not explained in detail within this protocol.
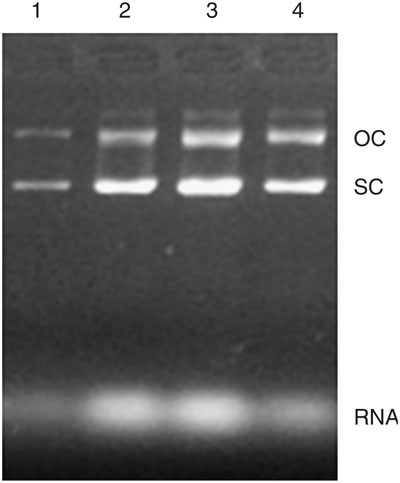
The following plasmids have been extracted using this protocol: pcDNA3, pGEX-4T-1, pVAX1 and pcD-VP1. The latter, encoding a capsid protein VP1 (639 bp) of foot and mouth disease virus (FMDV; described previously 22 ), was extensively used during the optimization of this protocol.
Adjust the flow rate using a stop watch so that the bacteria pass through the heated coil within 20 s. We have found this to be an optimal time period for the thermal lysis of cells; however, other times may be tested if an optimal yield can not be obtained. As indicated in Figure 2, the temperatures of 70 °C and 80 °C achieved a higher yield of plasmids with a minimum contamination with genomic DNA. However, 70 °C is probably the most appropriate setting in terms of the balance between energy costs and the quality of plasmids produced. An immediate connection from the heating coil to the coil submerged in ice should be used to avoid excess lysis.
To obtain perfect floc precipitates, do not vigorously shake the mixture of plasmid after adding isopropyl alcohol. If a high yield of plasmid is obtained from the precipitation, the incubation time can be reduced, as appropriate, and white floc can be immediately collected by passing through the 200 mesh nylon filter.
Dipping the bucket into 70% ethanol several times can enable effective rinsing. This will remove contamination as the dipping action will disassociate substances from the plasmid floc. Rinsing with 70% ethanol is important to eliminate impure substances, such as RNAs; sometimes, an additional rinse with chloroform before the 70% ethanol washes will remove some of the protein or lipid contamination.
Troubleshooting advice can be found in Table 2.
This work was supported, in part, by the China High Technology '863' Project (2004AA213102 and 2003AA241110), the China Key Technologies R&D program and a special research fund to B.W. provided by China Agricultural University. We would also like to thank Dr Jane Q.L. Yu for her assistance with the work, and Dr Terry Ng for his critical reading and valuable suggestions for the manuscript.